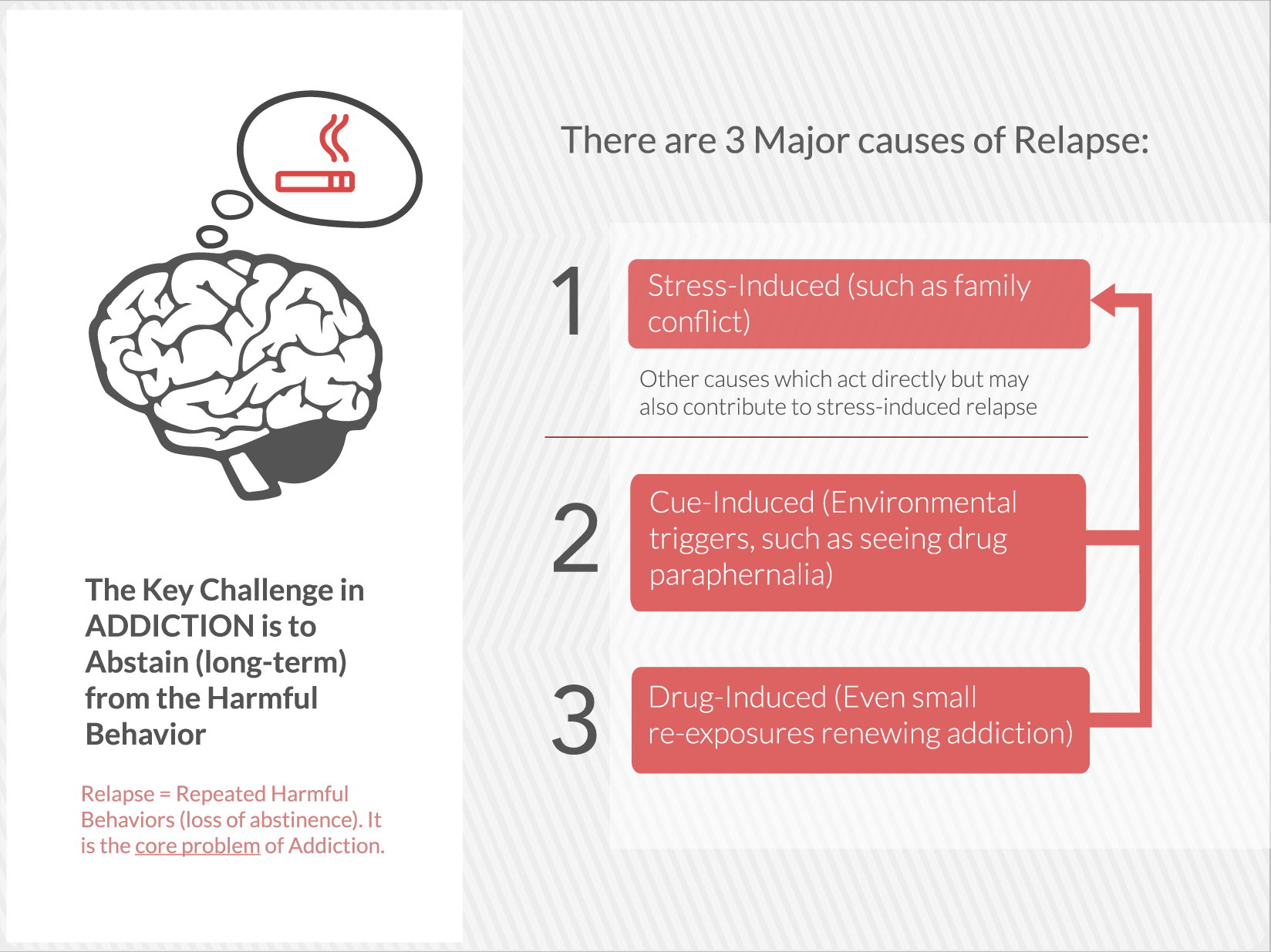
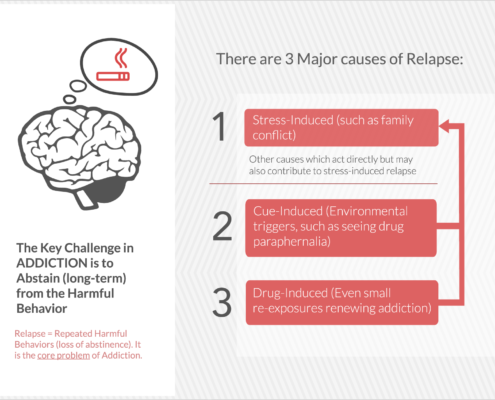
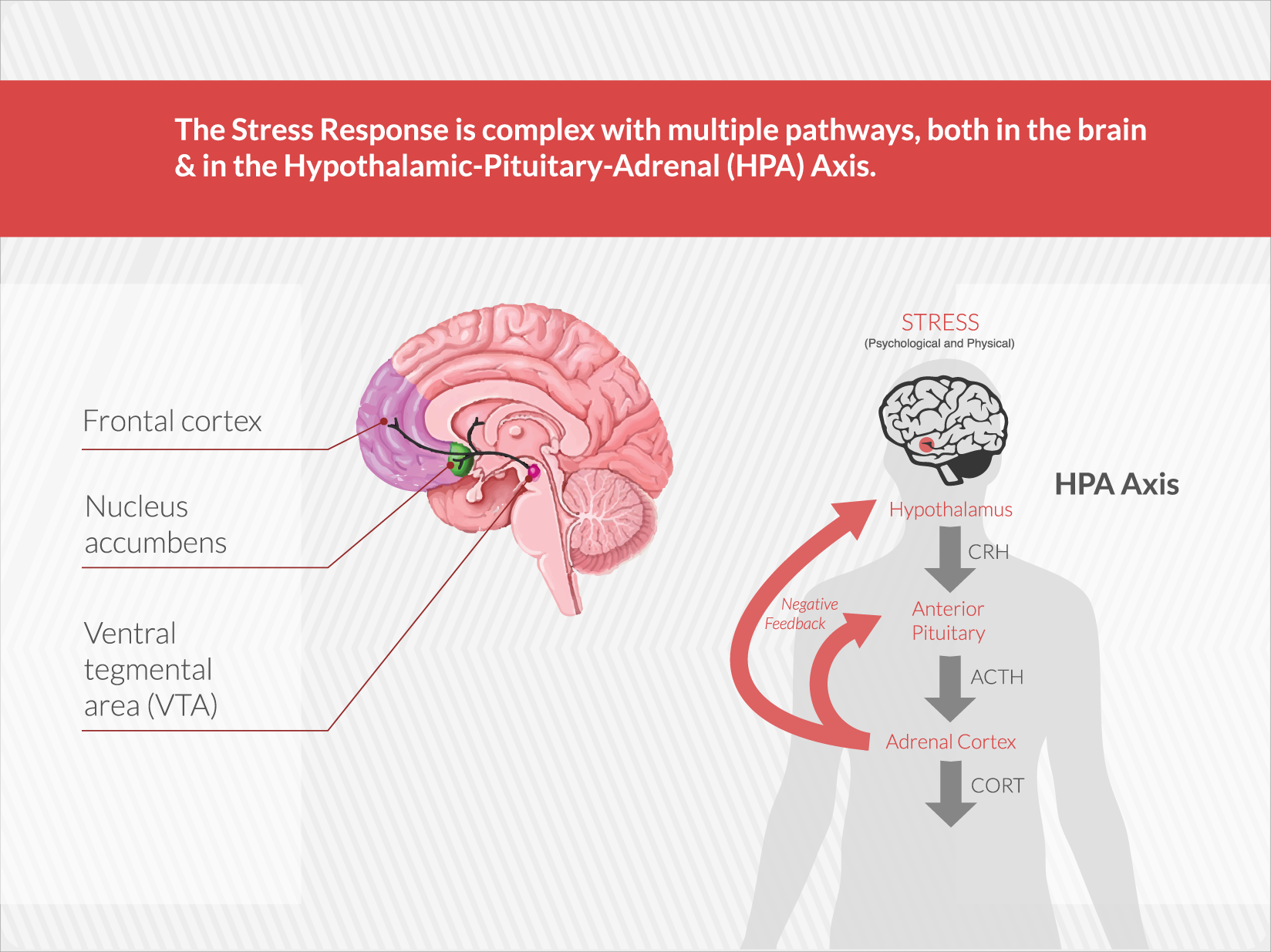
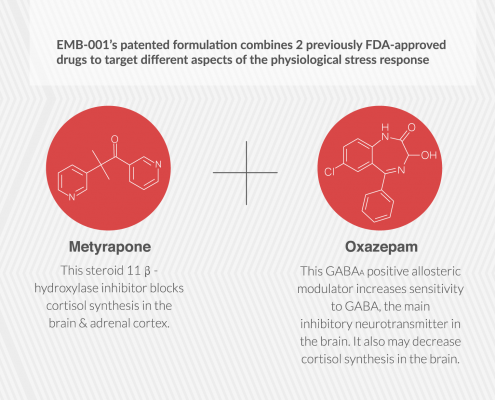
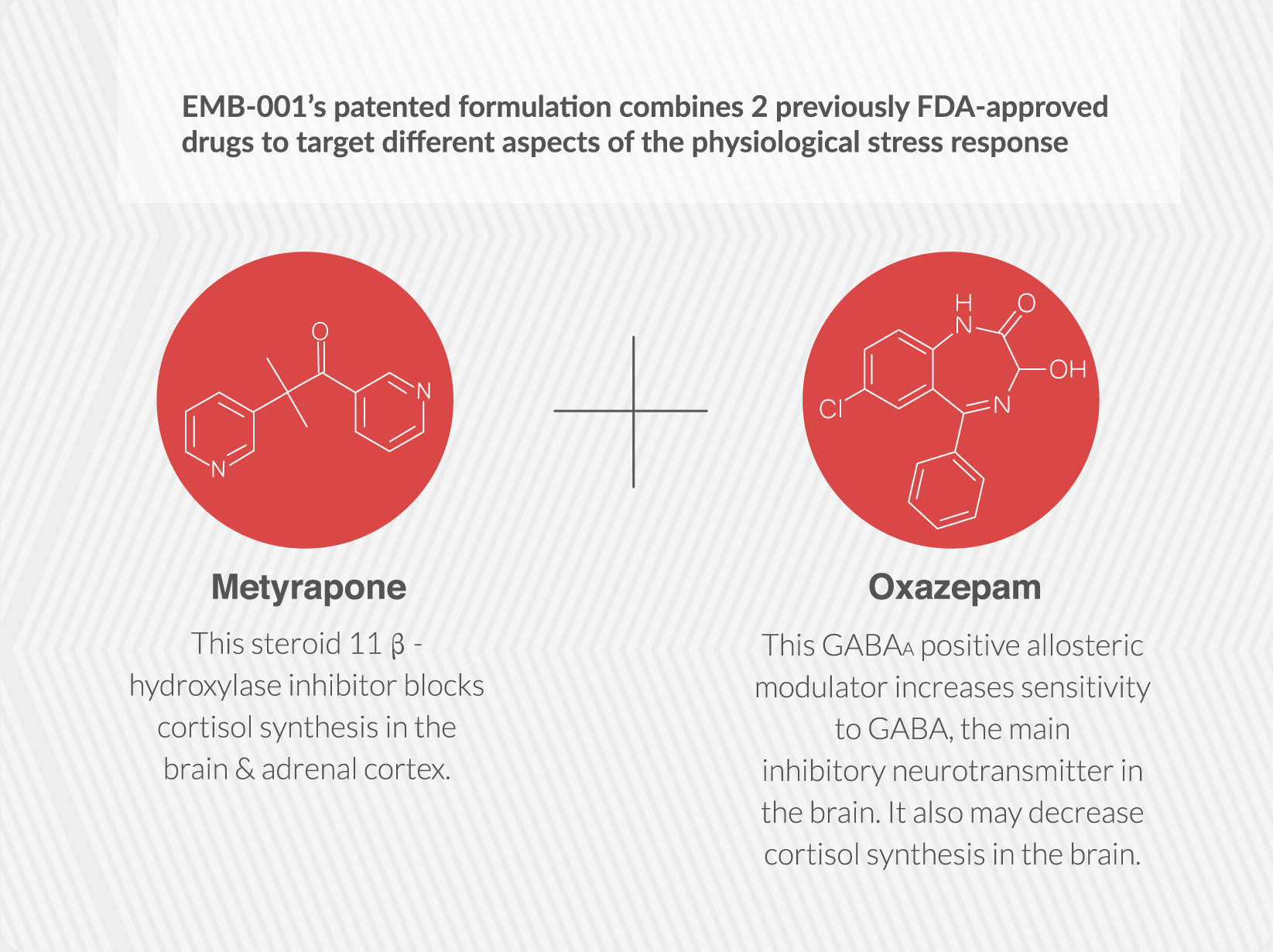
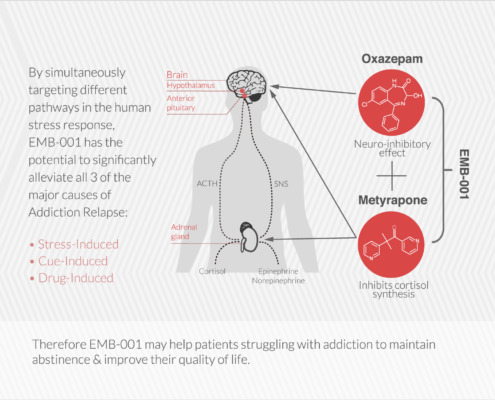
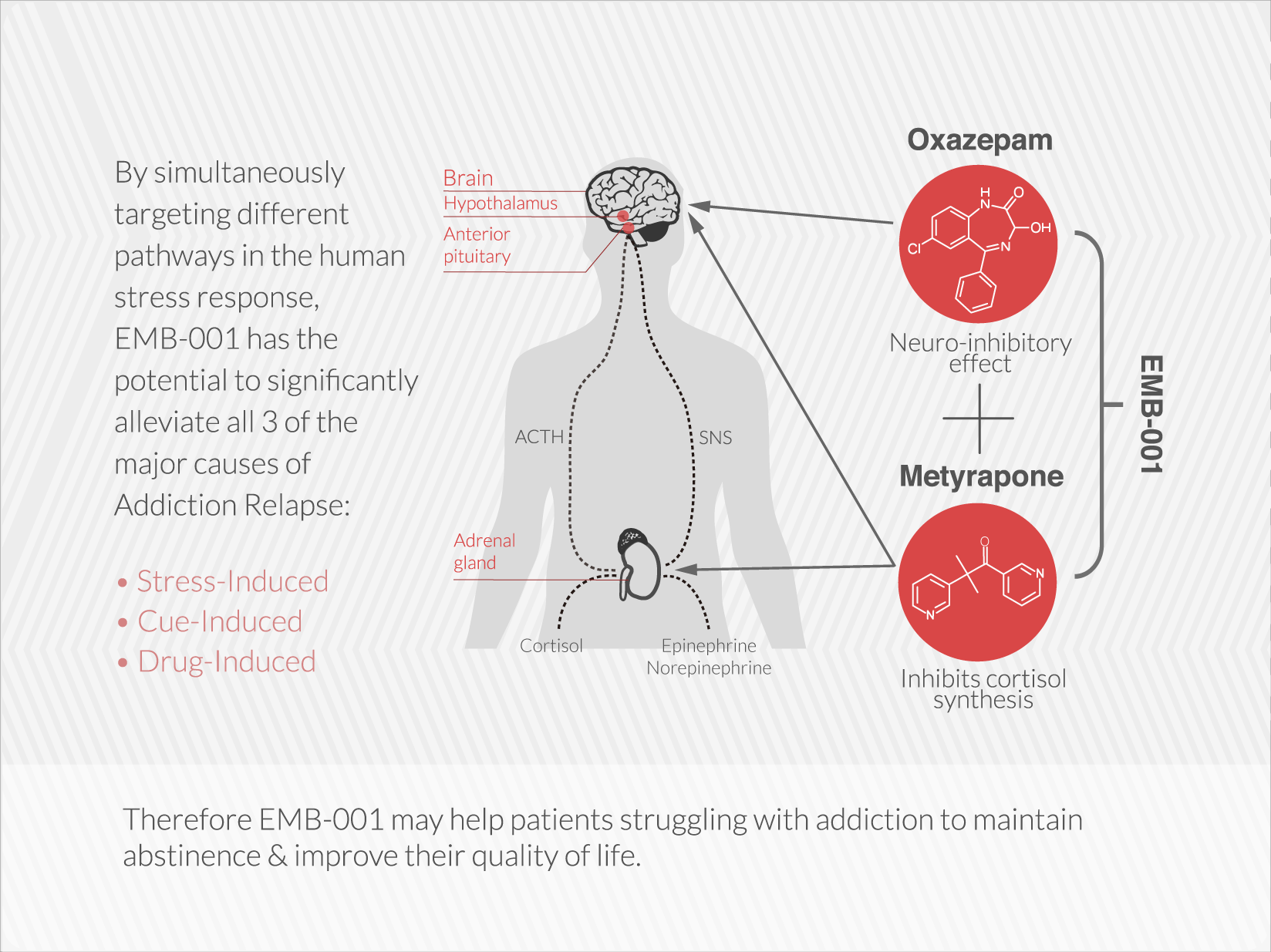
Embera is developing the first medication that treats addiction by moderating activity in the stress response system.
EMB-001 may potentially reduce the cravings and loss of control that drive addiction by uniquely targeting multiple pathways, to maximize potential efficacy more than either drug used alone. A therapy that breaks these barriers and results in long term abstinence and recovery would be a significant contribution to the treatment of a broad range of addictions.